Platform technologies have the ability to radically improve upon current products and generate completely novel products. In this sense, they open up new arenas for drug discovery and development, potentially increasing the number of therapeutic options for patients, says Brian Atwood, CEO of Cell Design Labs.
“Once a single compound or therapeutic has been generated and demonstrates a clinical benefit in patients, it is more likely this platform technology can successfully be applied to other therapeutic areas, derisking future compounds/products,” says Mr. Atwood.
Complex drugs by their very nature are challenging and costly to manufacture. This, in turn, translates into higher costs for patients and other payers, explains Ross Macdonald, PhD, Managing Director and Chief Executive Officer of Cynata Therapeutics. He says: “In order to provide safe and effective therapies at a reasonable price, it is necessary for the industry to develop manufacturing technologies that reduce costs and provide a consistent product.”
“While the initial investment may be larger, manufacturing costs will be lower over time as the manufacturing process is solidified,” agrees Mr. Atwood.
Despite the initial upfront costs, Dr. Macdonald says that platform technologies inevitably provide pragmatic solutions to production challenges, while yielding safer and more effective therapeutic products.
Drug Development & Delivery’s second annual report on platform technologies highlights some of these novel discoveries and describes how they are transforming drug development.
Precision NanoSystems: Controlled, Tuned & Fully Scalable Manufacturing of Nanomedicines
Nanoparticle encapsulation is gaining momentum as an effective drug delivery system, but traditional manufacturing methods are labor intensive, hard to reproduce, and difficult to scale up. Precision NanoSystems Inc. has developed a proprietary technology – the NanoAssemblrTM platform – for the rapid development of nanoparticles and seamless scale-up for clinical studies and commercial production.
This cartridge-based microfluidic system takes advantage of the highly predictable, time-invariant mixing offered by laminar flow to ensure rapid, controlled production of a variety of nanoparticles for the delivery of drugs or biological molecules.
“This standardization of processing allows the manufacture of nanoparticles with carefully defined characteristics, including chemical compositions, concentrations and drug/excipient ratios,” says James Taylor, CEO and Co-founder.
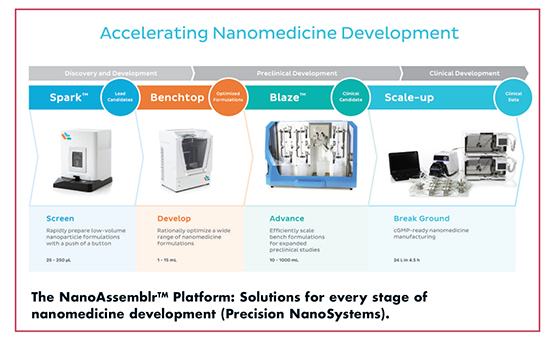
Parallelization is at the core of the NanoAssemblr technology, enabling throughput to be increased to meet the requirements of each phase of the drug development workflow without changing the reaction conditions. Precision NanoSystems offers four small footprint systems based on the same underlying microfluidic technology to match the various needs of the market. The smallest system in the family – the NanoAssemblr SparkTM – is designed for early-stage research and development activities, using disposable microfluidic cartridges to provide controlled and reproducible formulation of 25-250μl nanoparticle suspensions in less than 10 seconds. Once screening is complete, the NanoAssemblr BenchtopTM enables process development and optimization in 1-15-ml volumes. A simple, semi-disposable microfluidic cartridge offers flow rates up to 18 ml/min, and most nanoparticle formulations are completed in just 15-20 seconds, allowing more than 40 formulations to be prepared in one day, explains Mr. Taylor.
“The NanoAssemblr Blaze is designed to seamlessly scale NanoAssemblr Benchtop formulations up to one liter volumes. Using the same microfluidic mixer as the NanoAssemblr Benchtop, it employs continuous flow and parallelization to increase volumes and throughput, greatly reducing the need for process development compared to traditional methods,” says Mr. Taylor.
Finally, the largest system in the family – the NanoAssemblr Scale-Up – has been developed specifically for manufacturing clinical grade materials in the cGMP environment. Designed to be run in a clean room and customizable to meet customers’ specific requirements, it offers a completely flexible solution from early stage clinical trial manufacturing to commercial production. Mr. Taylor says: “This powerful technology is transforming the development and manufacturing of a range of nanoparticle formulations from a hit-and-miss affair to a standardized process, accelerating novel nanomedicines from the bench to the clinic.”
